Language and Labels
Database & Translations
Clinical Liability
Security & Product
WM helps hospitals and pharmacies owners meet the General Pharmaceutical Council (GPhC) and Care Quality Commission (CQC) expectations of providing equitable care and reasonable adjustments for patients with language communication barriers by providing translated and pictogram-enhanced medication information, reducing health inequalities and improves patient safety, experience, and adherence.
WM currently translates from English to the following 14 languages:
These languages are top commonly spoken first languages in the UK after English.
Only the directions of use, supplementary information and warnings are translated.
Yes, WM bilingual labels adhere to labelling guidance from Medicines and Healthcare products Regulatory Agency (MHRA) , NHS England and the Royal Pharmaceutical Society (RPS).
WM labels contain the following legal and clinical information:
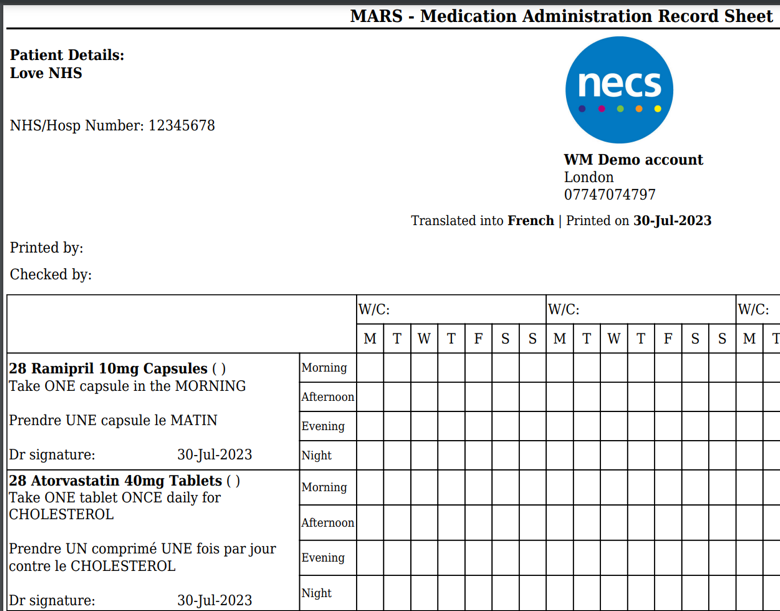
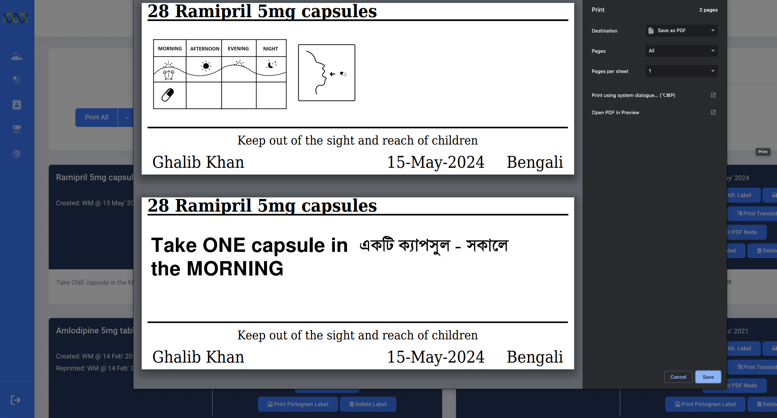
The bilingual labels look like your traditional English labels with an additional language.
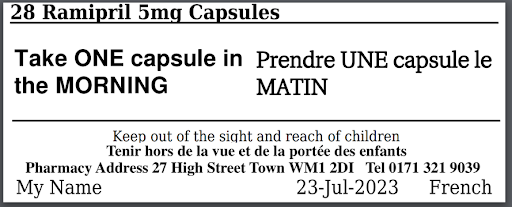
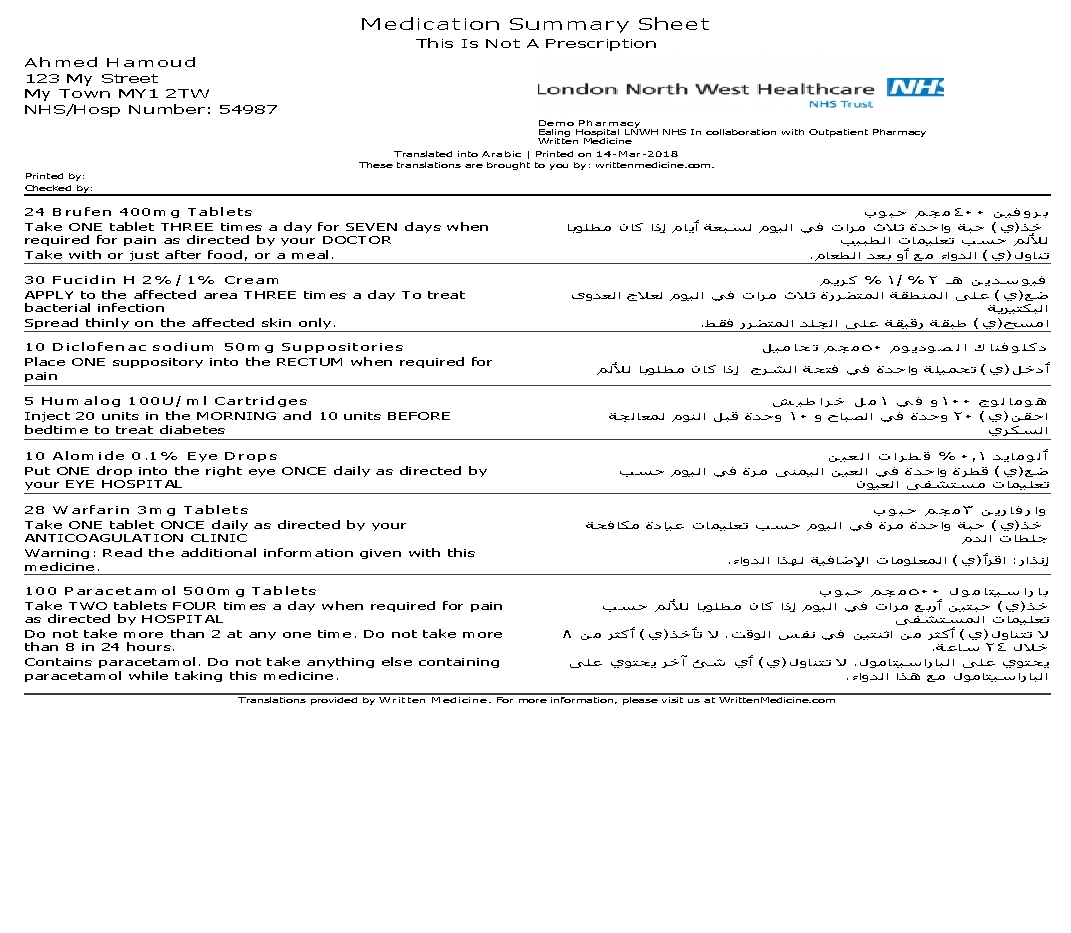
The bilingual medication information can also be printed as an A4 medication summary and Medication Administration Record (MAR) sheet.
MAR Charts comes in:
First Data Bank (FDB) is WM’s provider of medical products and devices that are linked to warnings and kept up-to-date monthly.
WM has developed its own proprietary dataset of 3,500 directions of use phrases in standardised English, known as Dose Syntax.
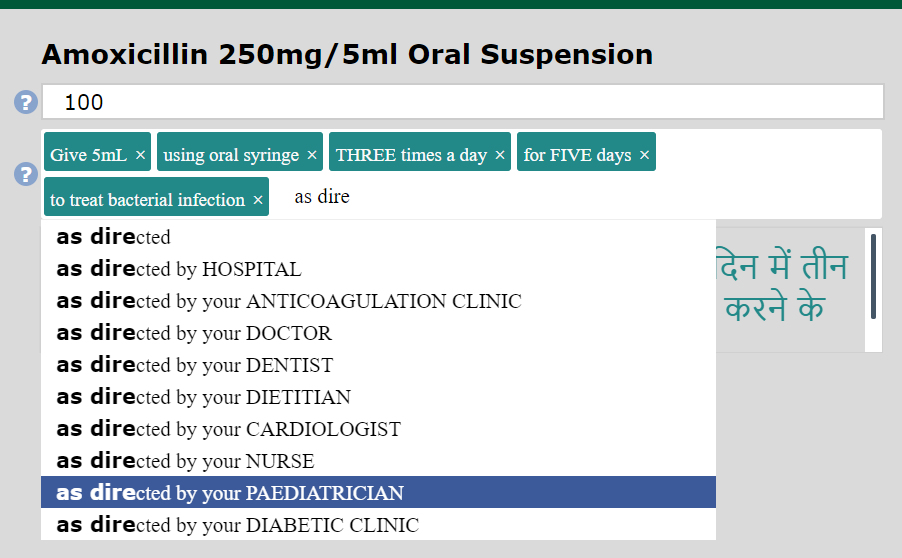
Periodically phrases are added and put through the language development process. If you cannot find a phrase on the system, you can submit a request to WM via;

Medicinal products and devices are updated automatically.
If a medicinal product is missing in the WM system, it can be added as a “Custom Drug” to generate a label and automatically submit a request to add.
You can also submit a request to WM via;
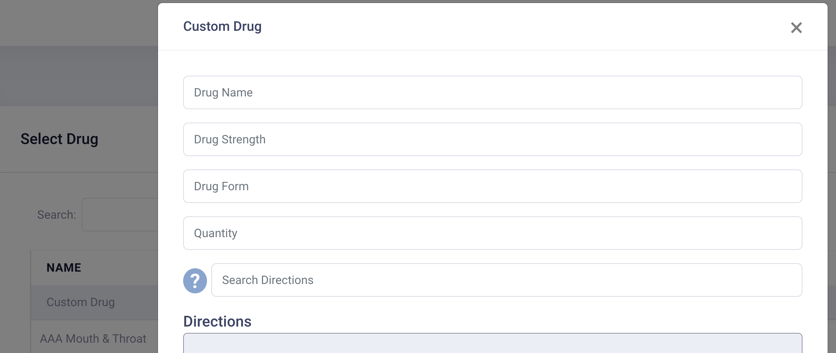
WM prioritises quality and safety seriously, developing a comprehensive four step language development and translation process with a standard operating procedure (SOP)*, incorporating co-production:
WM does not use Artificial Intelligence (AI) or Machine Learning (ML) when creating translations.
New languages are added upon customer request.
WM engages with all the UK regulatory and professional bodies who support innovations such as ours that improves patient safety and quality of care.
WM ensures that bilingual labels comply with the Human Medicines Regulations 2012, which allow information to be provided in multiple languages as long as it is identical to the English version and meets all regulatory requirements.
MHRA states- “The responsibility for any additional languages used would rest with the supplier of the information.”
WM is covered by a comprehensive professional indemnity and public and product liability insurance policy.This covers the translations and errors made around them, which includes the following summary;
Public & Product Liability - £10,000,000
The National Pharmacy Association (NPA) provides additional indemnity for community pharmacies using WM to cover bilingual pharmacy labels through their Professional Indemnity Insurance.
If you do not have indemnity with the NPA and would like to use WM, please share the details of your provider to make similar arrangements.
NHS Trusts using WM are indemnified through NHS Resolution. The pharmacy department should contact the Trust’s lead for Clinical Negligence Scheme for Trusts (CNST).
General Practises in England are indemnified through the NHS Resolution state indemnity scheme for general practice in England (The Clinical Negligence Scheme for General Practice).
As the supplier of translated information, WM is responsible for the service it provides, including the software. WM does not limit or exclude its liability for negligence if its service causes injury or death. WM also mitigates risk through arrangements with third parties, ensuring it is not liable for their negligence, breach of duty, or breach of contract.
WM ensures that all patient data is kept safe and secure within its system and databases in line with Data Protection Impact Assessment (DPIA).
WM is registered with the Information Commissioner Office (ICO) - ZA183249
WM is registered with NHS DSP Toolkit - Organisation code: 8JA19
WM requests the client to add the following information to generate a patient:
The data retention period IN WM is fully configurable to meet your obligations, as required by the NHS Records Management Code of Practice 2021. We recommend a minimum of two years, in line with the code of practice.
The data is owned by the client and can be downloaded in an encrypted file. Data remains available for up to 2 years after the contract ends. WM stores data in an encrypted database, accessible only via Two-Factor Authentication (2FA).
At the end of contract an offboarding process will be instigated and client data can be extracted via XML file.
Clients are responsible for ensuring the accuracy of patient data entered into the system. Data can be edited after a patient record is created. WM will not access or modify client data unless explicitly granted access by the client Super Administrator. Therefore, WM is not responsible for inaccuracies in client data.
WM is the data processor and does not have direct access to patient sensitive data. WM will not share information unless required by law and retains only necessary information for business practices.
WM utilises Amazon Web Services (AWS) cloud servers located in London, England.
WM encrypts all data between the client and its servers via the latest PCI 3.2 compliance, which requires either a Transport Layer Security (TLS) 1.2 or 1.3 for all communication. Data at rest on WM servers are stored in 256bit AES encryption.
Further information on DPIA can be requested
WM can integrate with third-party IT system suppliers across secondary care, primary care and community pharmacy via multiple methods, including open standards such as Fast Healthcare Interoperability Resources (FHIR), which the NHS supports.
The type of API integration used can differ depending on expected functionality. The most common type is Basic.
WM can provide a monthly database upload to system suppliers of:
Using WM will be intuitive, as the workflow is similar to the systems you already use in the pharmacy. WM anticipates it will take:
Login
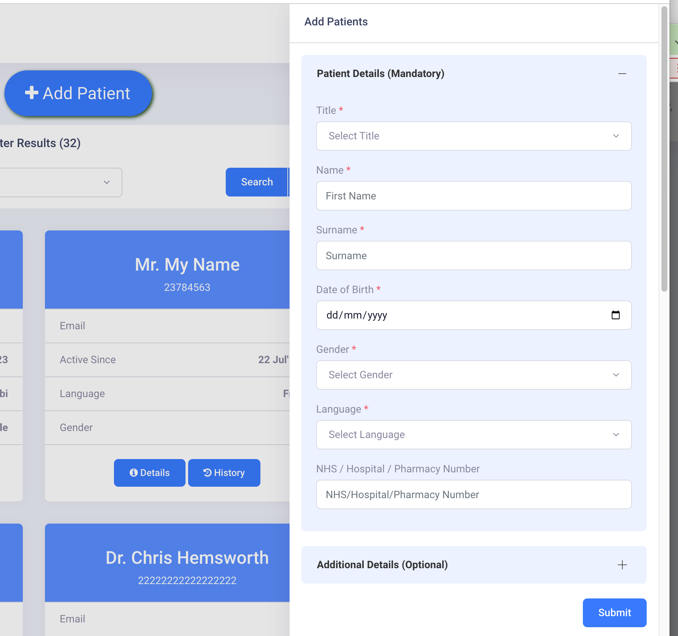
Adding a new patient
Adding the patient information can take 30 seconds.
If the patient already exists it shouldn’t take more than 15 seconds to search for them.
*The pharmacist can use the professional judgement not to add patient name.
Adding a new drug.
20 seconds
*This step will be skipped if it is already on their record.
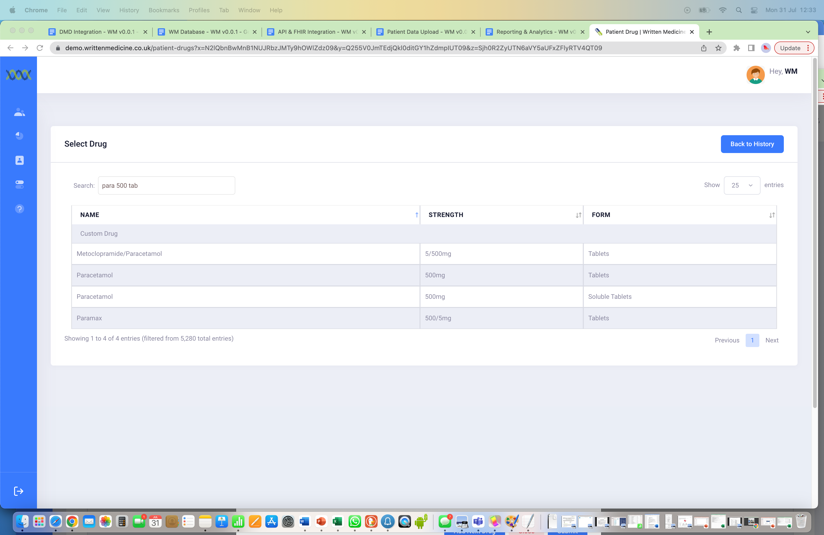
Adding directions of use
This process can take 30 seconds.
*This step will be skipped if it is already on their record.
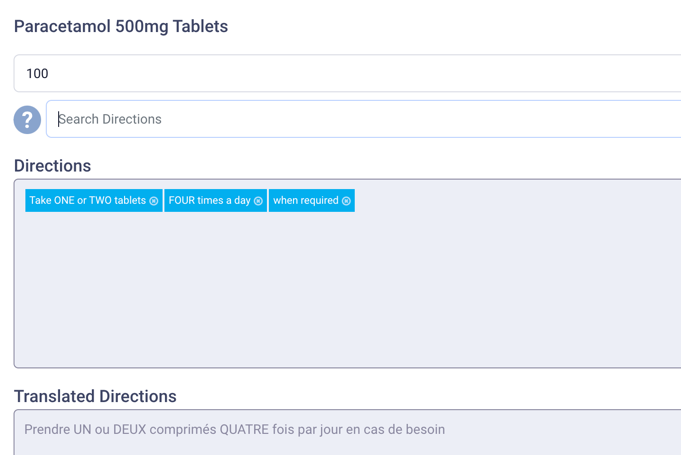
Selecting medicine and printing
This the final step should not take more than 15 seconds.
At this stage multiple medicines can be selected for printing.
WM utilises the existing hardware and software setup used by the client.
These are the technical requirements needed to utilise WM.
WM will ensure its service is available seamlessly. If you have any difficulties with the service, require technical support or need help, please contact WM by email (support@writtenmedicine.com) or the via Freshdesk icon.
Support is available Monday to Friday 9am to 5pm.

If you cannot find a phrase on the system or would like to recommend adding some, please submit a request to WM via:
If a medicinal product is missing in the WM system, it can be added as a “Custom Drug”.
Please contact WM and provide information about the missing medicinal product by email or via:
If a BNF cautionary and advisory warning is missing, please inform WM via the same process as above.
Please inform WM immediately and contact Ghalib Khan on 07747074797 or by email (support@writtenmedicine.com), mentioning;
WM will inform clients of any scheduled updates, works and downtime, 28 days in advance and will try to minimise it to a weekend or overnight outside of business hours.
For more information on what support is available please refer to WM Service Level Agreement (SLA).
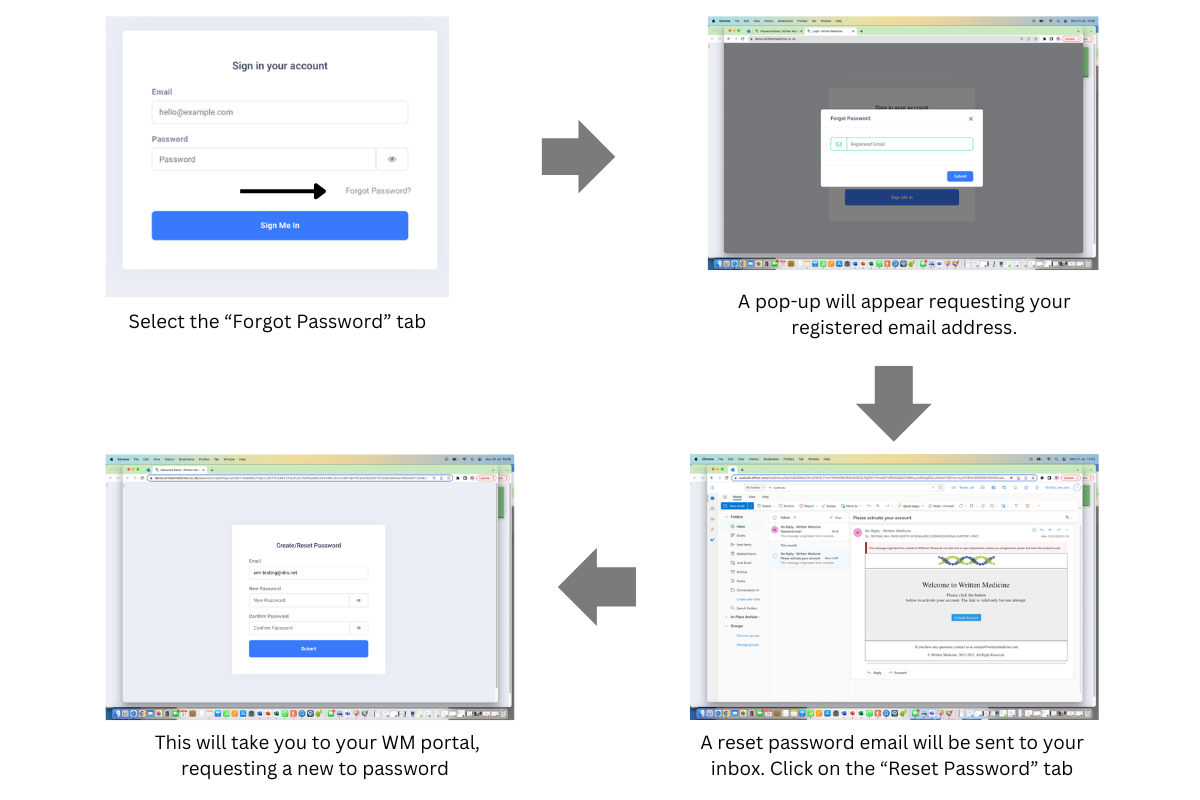
Users can reset their own passwords.
Reporting of the creation of the labels for the purpose of auditing the system is important in creating a trail to ensure that all best practices are adhered to within a healthcare system.
Based on the reporting done, proper analysis can take place to best inform supervisors and other senior staff on proper course of action, changes of practice, or other informed decisions. Reports can be printed or exported and sorted by labels and patients. This can be used for analysis or to save patient data once the pilot is complete.
The Reporting Module records each label generated with. There are two parts:
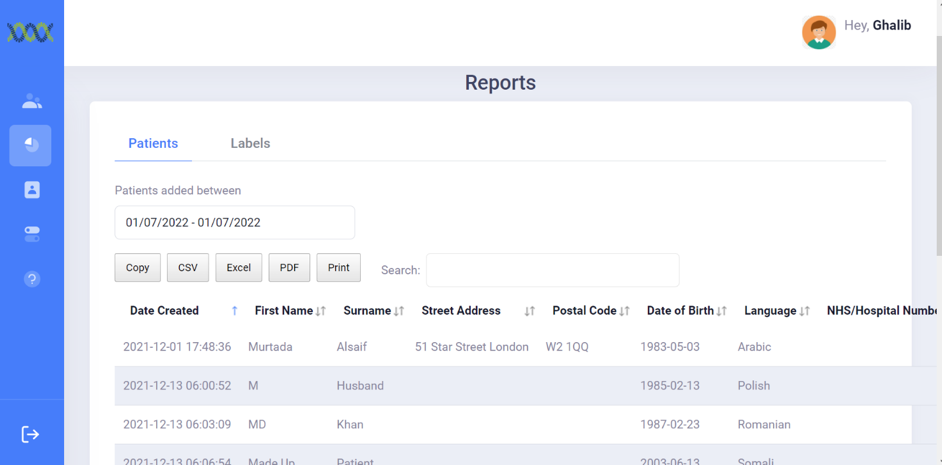
The data stored for the records patients:
The data stored for labels include:
For ease of use and analyses, Written Medicine provides an easy on-click solution to download the data in a convenient and easily read format. These include:
Every organisation will be given one-to-one training in-person and/or on MS Teams. WM also has;
The user guide will walk the client through how to use the WM system and how to troubleshoot any elements of the experience.
WM will have a training and education portal within your account, which will have;